Multiple Choice
Henry's Law constant is 0.034 mol/kg.bar and 0.0013 mol/kg.bar for CO2 and O2 respectively at 25 C.What pressure of O2 is required to achieve the same solubility as 0.218 bar of CO2?
A) 5.7 bar
B) 0.0083 bar
C) 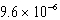 bar
bar
D) 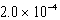 bar
bar
E) 0.18 bar
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: According to the National Institute of
Q2: Which of the following solutions would have
Q3: The vapor pressure of water at 90°C
Q5: _ are colloidal dispersions of one liquid
Q6: The Henry's law constant for O<sub>2</sub>
Q9: What mass of ethylene glycol,when mixed
Q10: The change in energy accompanying the
Q11: Assuming ideal behavior,which of the following aqueous
Q11: What is the vapor pressure at 20°C
Q54: Two nonpolar solvents,such as hexane and carbon