Multiple Choice
According to the National Institute of Standards webbook,the Henry's Law constant for N2 gas is 0.00060 mol/kg.bar at 25 C What is the Henry's law constant in units of mol/kg.atm? (1 bar = 0.9869 atm; 1 atm = 760 mmHg)
A) 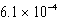 mol/kg.atm
mol/kg.atm
B) 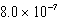 mol/kg.atm
mol/kg.atm
C) 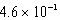 mol/kg.atm
mol/kg.atm
D) 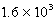 mol/kg.atm
mol/kg.atm
E) 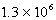 mol/kg.atm
mol/kg.atm
Correct Answer:

Verified
Correct Answer:
Verified
Q2: Which of the following solutions would have
Q3: The vapor pressure of water at 90°C
Q4: Henry's Law constant is 0.034 mol/kg.bar
Q5: _ are colloidal dispersions of one liquid
Q6: The Henry's law constant for O<sub>2</sub>
Q9: What mass of ethylene glycol,when mixed
Q10: The change in energy accompanying the
Q11: Assuming ideal behavior,which of the following aqueous
Q11: What is the vapor pressure at 20°C
Q54: Two nonpolar solvents,such as hexane and carbon