Multiple Choice
Which of the following conclusions concerning the concentration-time plot provided below is/are correct?
1) The concentration of substance D is decreasing over time.
2) The instantaneous reaction rate at point A is less than the instantaneous reaction rate at point B.
3) Substance D is a product of the reaction. 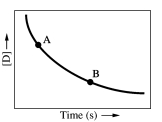
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 1,2,and 3
Correct Answer:

Verified
Correct Answer:
Verified
Q8: Termolecular elementary steps are rare.Why?
Q53: Consider the exothermic combustion of coal.Which of
Q54: The decomposition of formic acid follows
Q56: Given the initial rate data for
Q57: A second-order reaction starts with an
Q59: The elementary steps for the catalyzed
Q60: For a chemical reaction,the activation energy for
Q61: For the hypothetical reaction aA
Q62: The reaction A <span class="ql-formula"
Q63: Calculate the activation energy,E<sub>a</sub>,for<br>N<sub>2</sub>O<sub>5</sub>(g) <span class="ql-formula"