For the Hypothetical Reaction AA Products,the Experimental Data Showed the Following Behavior (Below)
Multiple Choice
For the hypothetical reaction aA products,the experimental data showed the following behavior (below) .What is the reaction order with respect to reactant A? 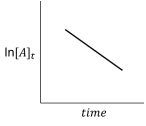
A) zero order
B) first order
C) second order
D) third order
E) fourth order
Correct Answer:

Verified
Correct Answer:
Verified
Q56: Given the initial rate data for
Q57: A second-order reaction starts with an
Q58: Which of the following conclusions concerning the
Q59: The elementary steps for the catalyzed
Q60: For a chemical reaction,the activation energy for
Q62: The reaction A <span class="ql-formula"
Q63: Calculate the activation energy,E<sub>a</sub>,for<br>N<sub>2</sub>O<sub>5</sub>(g) <span class="ql-formula"
Q65: The pre-exponential,A,in the Arrhenius equation is called
Q65: Which of the following is
Q66: Given the initial rate data for