Assume the Reaction Below
2 NO(g)+ O2(g) 2 NO2(g)
Proceeds Via the Following Rate Expression:
Which
Multiple Choice
Assume the reaction below
2 NO(g) + O2(g) 2 NO2(g)
Proceeds via the following rate expression: 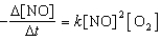
Which of the following statements concerning the above chemical reaction and rate equation is/are CORRECT?
1) The reaction is second-order with respect to NO.
2) The rate of disappearance of O2 is two times the rate of appearance of NO2.
3) According to the balanced chemical equation,the reaction is fifth-order overall.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 3
E) 2 and 3
Correct Answer:

Verified
Correct Answer:
Verified
Q13: The reaction kinetics for a certain reaction
Q31: Draw the reaction coordinate diagram for an
Q34: In a reaction coordinate diagram,reacting molecules are
Q62: Carbon dating may be used to date
Q68: The Arrhenius equation, <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg" alt="The Arrhenius
Q69: The decomposition of phosphine,PH<sub>3</sub>,follows first-order kinetics.<br>4
Q70: For the overall reaction<br>2A + B
Q71: For a certain overall third-order reaction
Q72: The rate law for a reaction
Q78: For a certain reaction of the