Multiple Choice
The Arrhenius equation, 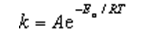 ,relates the rate constant of reaction and temperature.A plot of ____ versus 1/T will yield a straight line with a slope of -Ea/R.
,relates the rate constant of reaction and temperature.A plot of ____ versus 1/T will yield a straight line with a slope of -Ea/R.
A) k2/k1
B) -Ea
C) ln(k)
D) 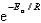
E) 1/RT
Correct Answer:

Verified
Correct Answer:
Verified
Q63: Calculate the activation energy,E<sub>a</sub>,for<br>N<sub>2</sub>O<sub>5</sub>(g) <span class="ql-formula"
Q65: Which of the following is
Q65: The pre-exponential,A,in the Arrhenius equation is called
Q66: Given the initial rate data for
Q67: The rate constant for a first-order
Q69: The decomposition of phosphine,PH<sub>3</sub>,follows first-order kinetics.<br>4
Q70: For the overall reaction<br>2A + B
Q71: For a certain overall third-order reaction
Q72: The rate law for a reaction
Q73: Assume the reaction below<br>2 NO(g)+ O<sub>2</sub>(g)