Multiple Choice
Write the expression for K for the reaction below.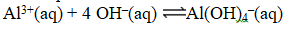
A) 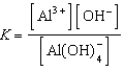
B) 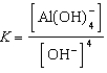
C) 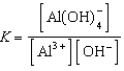
D) 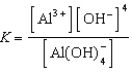
E) 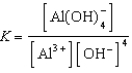
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q16: In 1913,the Haber-Bosch process was patented.The product
Q72: Excess Ag<sub>2</sub>SO<sub>4</sub>(s)is placed in water at
Q73: Consider the following equilibrium:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg" alt="Consider the
Q74: At 800 K,the equilibrium constant,K<sub>p</sub>,for the
Q75: Given the equilibrium constants for the
Q76: Consider the following equilibrium at 25°C:<br>2ICl(g)
Q78: If K<sub>c</sub> = 0.152 for A<sub>2</sub> +
Q79: What is the expression for K<sub>c</sub> for
Q80: A sample of solid NH<sub>4</sub>NO<sub>3</sub> was placed
Q81: When the pressure of an equilibrium mixture