Multiple Choice
At 800 K,the equilibrium constant,Kp,for the following reaction is
3.2 10-7.2 H2S(g) 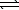 2 H2(g) + S2(g)
2 H2(g) + S2(g)
A reaction vessel at 800 K initially contains 3.00 atm of H2S.If the reaction is allowed to equilibrate,what is the equilibrium pressure of S2?
A) 8.5 10-5 atm
B) 6.2 10-3 atm
C) 9.0 10-3 atm
D) 1.1 10-2 atm
E) 1.4 10-2 atm
Correct Answer:

Verified
Correct Answer:
Verified
Q69: Write the expression for K<sub>p</sub> for the
Q70: A 3.00-liter flask initially contains 3.00
Q71: A 2.50-mol sample of HI is
Q72: Excess Ag<sub>2</sub>SO<sub>4</sub>(s)is placed in water at
Q73: Consider the following equilibrium:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg" alt="Consider the
Q75: Given the equilibrium constants for the
Q76: Consider the following equilibrium at 25°C:<br>2ICl(g)
Q77: Write the expression for K for the
Q78: If K<sub>c</sub> = 0.152 for A<sub>2</sub> +
Q79: What is the expression for K<sub>c</sub> for