Multiple Choice
At 25 C,the decomposition of dinitrogen tetraoxide
N2O4(g) 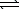 2 NO2(g)
2 NO2(g)
Has an equilibrium constant (Kp) of 0.144.At equilibrium,the total pressure of the system is 0.0758 atm.What is the partial pressure of each gas?
A) 0.0745 atm NO2(g) and 0.0385 N2O4(g)
B) 0.0549 atm NO2(g) and 0.0209 N2O4(g)
C) 0.0531 atm NO2(g) and 0.0227 N2O4(g)
D) 0.0502 atm NO2(g) and 0.0256 N2O4(g)
E) 0.0381 atm NO2(g) and 0.0377 N2O4(g)
Correct Answer:

Verified
Correct Answer:
Verified
Q59: If a stress is applied to an
Q59: Which of the following statements is/are CORRECT?<br>1)Product
Q60: Consider the reaction <br>A(aq) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg"
Q61: Ozone is formed from oxygen.3 O<sub>2</sub>(g)
Q62: The standard enthalpy of formation of ammonia
Q65: For which one of the following reactions
Q66: What is the balanced equation for the
Q67: Given the following chemical equilibrium,<br>COCl<sub>2</sub>(g) <img
Q68: Consider the following reaction:<br><img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg" alt="Consider the
Q69: Write the expression for K<sub>p</sub> for the