Essay
The standard enthalpy of formation of ammonia is -46.1 kJ/mol.
1/2 N2(g)+ 3/2 H2(g) 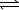 NH3(g)
NH3(g)
Commercially,the reaction is carried out at high temperatures.Using your knowledge of kinetics and equilibrium,explain an advantage and a disadvantage of synthesizing ammonia at high temperatures.
Correct Answer:

Verified
The rate of formation of ammonia is negl...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q57: Which of the following statements is/are CORRECT?<br>1)For
Q58: Nitrosyl bromide decomposes according to the
Q59: Which of the following statements is/are CORRECT?<br>1)Product
Q59: If a stress is applied to an
Q60: Consider the reaction <br>A(aq) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg"
Q61: Ozone is formed from oxygen.3 O<sub>2</sub>(g)
Q64: At 25 <sup> <span class="ql-formula" data-value="\circ"><span
Q65: For which one of the following reactions
Q66: What is the balanced equation for the
Q67: Given the following chemical equilibrium,<br>COCl<sub>2</sub>(g) <img