Multiple Choice
What is the equilibrium pH of an initially 0.64 M solution of the monoprotic acid benzoic acid at 25°C (Ka = 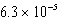 ) ?
) ?
A) 2.20
B) 7.00
C) 1.90
D) 12.10
E) 5.10
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q6: What is the equilibrium hydronium ion concentration
Q7: What is the pH of a
Q8: In the reaction<br>CuO(s)+ CO<sub>2</sub>(g) <span class="ql-formula"
Q9: What is the OH<sup>-</sup> concentration of
Q10: What is the pH of a
Q12: Calculate the pH of the following aqueous
Q13: The pH of a solution of
Q14: Which equation depicts aqueous hydrogen sulfide behaving
Q15: What is the H<sub>3</sub>O<sup>+</sup> concentration in
Q16: Carbonic acid is a diprotic acid,H<sub>2</sub>CO<sub>3</sub>,with