Multiple Choice
What is the equilibrium hydronium ion concentration of an initially 5.4 M solution of hypochlorous acid,HOCl,at 25°C (Ka = 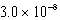 ) ?
) ?
A) 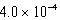 M
M
B) 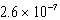 M
M
C) 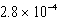 M
M
D) 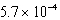 M
M
E) 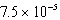 M
M
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q2: What is the hydronium-ion concentration in
Q4: Which of the following statements is INCORRECT?<br>A)
Q7: What is the pH of a
Q8: In the reaction<br>CuO(s)+ CO<sub>2</sub>(g) <span class="ql-formula"
Q9: What is the OH<sup>-</sup> concentration of
Q10: What is the pH of a
Q11: What is the equilibrium pH of an
Q13: Rank H<sub>3</sub>PO<sub>4</sub>,H<sub>2</sub>PO<sub>4</sub><sup>-</sup>,and HPO<sub>4</sub><sup>2-</sup> in order of increasing
Q78: All of the following compounds are acids
Q80: When a Lewis acid and a Lewis