Multiple Choice
Which acid-base combination is depicted by this titration curve? The dot on the curve is located at the titrant volume where the titration solution pH equals 7. 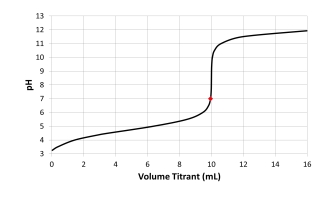
A) Titration of a weak acid with a strong base.
B) Titration of a strong acid with a strong base.
C) Titration of a weak base with a strong acid.
D) Titration of a strong base with a strong acid.
E) Not enough information provided.
Correct Answer:

Verified
Correct Answer:
Verified
Q29: The K<sub>sp</sub> of BaSO<sub>4</sub> is 1.1
Q30: What is the molar solubility of
Q31: What is the concentration of silver(I)ion
Q32: What is the pH of a
Q33: What is the minimum concentration of
Q36: An impure sample of sodium carbonate,Na<sub>2</sub>CO<sub>3</sub>,is titrated
Q37: How many moles of solid NaF
Q38: A 50.00-mL solution of 0.0729 M
Q39: What is the pH of a
Q45: To make a buffer with a pH