Multiple Choice
An impure sample of sodium carbonate,Na2CO3,is titrated with 0.113 M HCl according to the reaction below.
2 HCl(aq) + Na2CO3(aq) 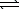 CO2(g) + H2O(
CO2(g) + H2O( 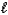 ) + 2 NaCl(aq)
) + 2 NaCl(aq)
What is the percent of Na2CO3 in a 0.613 g sample if the titration requires 26.14 mL of HCl? The molar mass of Na2CO3 is 106.0 g/mol.
A) 0.295%
B) 15.7%
C) 25.5%
D) 51.1%
E) 67.9%
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q31: What is the concentration of silver(I)ion
Q32: What is the pH of a
Q33: What is the minimum concentration of
Q34: Which acid-base combination is depicted by this
Q37: How many moles of solid NaF
Q38: A 50.00-mL solution of 0.0729 M
Q39: What is the pH of a
Q40: An acid-base equilibrium system is created by
Q41: What mass of sodium hydroxide must
Q45: To make a buffer with a pH