Multiple Choice
What is the solubility product expression for Fe(OH) 3?
A) Ksp = 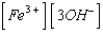
B) Ksp = 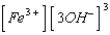
C) Ksp = 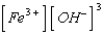
D) Ksp = 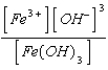
E) Ksp = 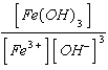
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q17: What is the pH of a
Q18: When a weak base is titrated with
Q19: What is the value of the
Q20: When mixed in appropriate amounts,each of the
Q21: What is the effect of adding NaOH(aq)to
Q23: What is the molar solubility of
Q24: Suppose 50.00 mL of 2.0
Q25: What is the minimum mass of
Q26: Given the following equilibrium constants,<br>Cd(IO<sub>3</sub>)<sub>2</sub> K<sub>sp</sub> =
Q27: An aqueous solution contains 0.010 M