Multiple Choice
Given the following equilibrium constants,
Cd(IO3) 2 Ksp = 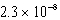
Cd(NH3) 42+ Kf = 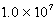
Determine K for the dissolution of the sparingly soluble salt Cd(IO3) 2 in aqueous ammonia (shown below) .Cd(IO3) 2(s) + 4NH3(aq)  Cd(NH3) 42+(aq) + 2IO3-(aq)
Cd(NH3) 42+(aq) + 2IO3-(aq)
A) 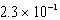
B) 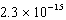
C) 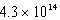
D) 4.3
E) 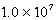
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q21: What is the effect of adding NaOH(aq)to
Q22: What is the solubility product expression for
Q23: What is the molar solubility of
Q24: Suppose 50.00 mL of 2.0
Q25: What is the minimum mass of
Q27: An aqueous solution contains 0.010 M
Q28: A 25.00-mL sample of propionic acid,HC<sub>3</sub>H<sub>5</sub>O<sub>2</sub>,of unknown
Q29: The K<sub>sp</sub> of BaSO<sub>4</sub> is 1.1
Q30: What is the molar solubility of
Q31: What is the concentration of silver(I)ion