Multiple Choice
Calculate the molar concentration of uncomplexed Zn2+(aq) in a solution that contains 0.24 M Zn(NH3) 42+ and 0.3671 M NH3 at equilibrium.Kf for Zn(NH3) 42+ is 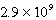 .
.
A) 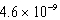 M
M
B) 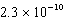 M
M
C) 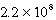 M
M
D) 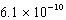 M
M
E) 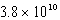 M
M
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q1: How many moles of HCl must be
Q2: A 25.0 mL sample of 0.200
Q3: What mass of solid NaCH<sub>3</sub>CO<sub>2</sub> (molar
Q4: The K<sub>sp</sub> of Ca(OH)<sub>2</sub> is 5.5
Q6: Hyperventilation can cause your blood pH to
Q8: For a monoprotic acid titration,the _ point
Q9: What is the pH of the
Q10: A 4.0 <span class="ql-formula"
Q11: What is the pH of a
Q21: Why do salts containing basic anions have