Multiple Choice
An SHE electrode has been assigned a standard reduction potential,E ,of 0.00 Volts.Which reaction occurs at this electrode?
A) 2 H2O( 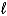 ) + 2 e- H2(g) + 2 OH-(aq)
) + 2 e- H2(g) + 2 OH-(aq)
B) O2(g) + 4 e- 2 O2-(aq)
C) Hg2Cl2(s) + 2 e- 2 Hg( 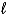 ) + 2 Cl-(aq)
) + 2 Cl-(aq)
D) Li+(aq) + e- Li(s)
E) 2 H+(aq) + 2 e- H2(g)
Correct Answer:

Verified
Correct Answer:
Verified
Q7: The following reaction occurs spontaneously.<br>2 Fe(s)+
Q9: Which of the following statements is/are CORRECT?<br>1)A
Q10: Calculate E<sub>cell</sub> for the following electrochemical cell
Q11: Al<sup>3+</sup> is reduced to Al(s)at an
Q13: Write balanced reduction and oxidation half-reactions for
Q14: Calculate <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg" alt=" Calculate
Q15: What is the standard cell potential
Q16: Consider the following half-reactions:<br>Cl<sub>2</sub>(g)+ 2 e<sup>-</sup>
Q17: Which of the following are standard conditions
Q58: Explain the function of a salt bridge