Multiple Choice
What is the standard cell potential ( 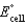 ) for the following below,
) for the following below,
2Ag(s) + Pb2+(aq) 2Ag+(aq) + Pb(s)
Given the following standard reduction potentials.
Ag+(aq) + e- Ag(s) E =0.8 V
Pb2+(aq) +2e- Pb(s) E =-0.126 V
A) -0.926 V
B) 0.926 V
C) -1.726 V
D) 1.726 V
E) 1.474 V
Correct Answer:

Verified
Correct Answer:
Verified
Q10: Calculate E<sub>cell</sub> for the following electrochemical cell
Q11: Al<sup>3+</sup> is reduced to Al(s)at an
Q12: An SHE electrode has been assigned
Q13: Write balanced reduction and oxidation half-reactions for
Q14: Calculate <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg" alt=" Calculate
Q16: Consider the following half-reactions:<br>Cl<sub>2</sub>(g)+ 2 e<sup>-</sup>
Q17: Which of the following are standard conditions
Q18: For the cell reaction <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg" alt="For
Q19: Calculate E°<sub>cell</sub> for the cell for
Q20: Given the following two half-reactions,write the