Multiple Choice
Calculate 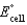 for the electrochemical cell below,
for the electrochemical cell below,
Pb(s) |PbCl2(s) | Cl-(aq,1.0 M) || Fe3+(aq,1.0 M) ,Fe2+(aq,1.0 M) | Pt(s)
Given the following reduction half-reactions.
Pb2+(aq) + 2 e- Pb(s) E = -0.126 V
PbCl2(s) + 2 e- Pb(s) + 2 Cl-(aq) E = -0.267 V
Fe3+(aq) + e- Fe2+(aq) E = +0.771 V
Fe2+(aq) + e- Fe(s) E = -0.44 V
A) -0.504 V
B) -0.062 V
C) +0.504 V
D) +1.038 V
E) +1.604 V
Correct Answer:

Verified
Correct Answer:
Verified
Q46: Which of the following species are likely
Q47: What half-reaction occurs at the cathode
Q48: Calculate E<sub>cell</sub> for the following electrochemical
Q49: When the following oxidation-reduction reaction in
Q50: According to the following cell notation,which species
Q52: What is a correct cell notation
Q53: If electric current is passed through a
Q54: What is the balanced spontaneous reaction
Q55: Which of the following statements is true
Q56: The cell potential of the following