Multiple Choice
The following has a potential of 0.92 V: 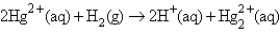 If the concentrations of the ions were 1.0 M and the pressure of H2 were 1.0 atm,then E for the half-reaction
If the concentrations of the ions were 1.0 M and the pressure of H2 were 1.0 atm,then E for the half-reaction 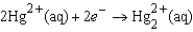
Would be
A) 0.46 V
B) -0.46 V
C) 0.92 V
D) -0.92 V
E) none of these
Correct Answer:

Verified
Correct Answer:
Verified
Q60: If the value of E°<sub>cell</sub> is <img
Q61: All of the following statements concerning voltaic
Q62: Which of the following reactions would
Q63: Calculate the cell potential,at 25 <sup>
Q64: How many moles of electrons are
Q66: The standard cell potential of the following
Q67: Write a balanced half-reaction for the
Q68: When the following oxidation-reduction reaction in
Q69: Use the standard reduction potentials below
Q70: Given: <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4499/.jpg" alt=" Given: