Multiple Choice
If the value of E°cell is 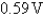 for the reaction
for the reaction 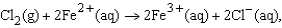
What is the value of E°cell for 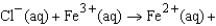
½ Cl2(g) ?
A) 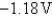
B) 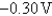
C) 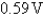
D) 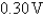
E) 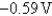
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q55: Which of the following statements is true
Q56: The cell potential of the following
Q57: Calculate E<sub>cell</sub> for the following electrochemical
Q58: What is the pH of the
Q59: What charge,in coulombs,is required to deposit
Q61: All of the following statements concerning voltaic
Q62: Which of the following reactions would
Q63: Calculate the cell potential,at 25 <sup>
Q64: How many moles of electrons are
Q65: The following has a potential of