Multiple Choice
What is the value of the reaction quotient,Q,for the voltaic cell constructed from the following two half-reactions when the Zn2+ concentration is 0.0120 M and the Ag+ concentration is 1.25 M? 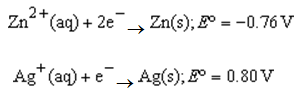
A) 9.60 10-3
B) 7.68 10-3
C) 104
D) 1.25 10-2
E) 130
Correct Answer:

Verified
Correct Answer:
Verified
Q39: Calculate the equilibrium constant for the
Q40: Balance the following half-reaction occurring in
Q41: How many electrons are transferred in the
Q42: Which of the following is true
Q43: For the electrochemical cell Zn(s)| Zn<sup>2+</sup> ||
Q45: What is the copper(II)-ion concentration at 25°C
Q46: Which of the following species are likely
Q47: What half-reaction occurs at the cathode
Q48: Calculate E<sub>cell</sub> for the following electrochemical
Q49: When the following oxidation-reduction reaction in