Multiple Choice
Calculate the equilibrium constant for the following reaction at 25 C,
2 IO3-(aq) + 5 Hg( 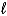 ) + 12 H+(aq) I2(s) + 5 Hg2+(aq) + 6 H2O(
) + 12 H+(aq) I2(s) + 5 Hg2+(aq) + 6 H2O( 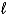 )
)
Given the following thermodynamic information.
IO3-(aq) + 6 H+(aq) + 5 e- I2(s) + 3 H2O( 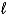 ) E = +1.20 V
) E = +1.20 V
Hg2+(aq) + 2 e- Hg( 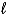 ) E = +0.86 V
) E = +0.86 V
A) 3 10-58
B) 6 105
C) 3 1011
D) 6 1028
E) 3 1057
Correct Answer:

Verified
Correct Answer:
Verified
Q34: Consider the following half-reactions:<br>Ag<sup>+</sup>(aq)+ e<sup>-</sup>
Q35: Write a balanced chemical equation for
Q36: Given:<br>Mn<sup>2+</sup>(aq)+ 2e<sup>-</sup> <span class="ql-formula" data-value="\to"><span
Q37: Balance the following oxidation-reduction occurring in
Q38: Which of the following statements is true
Q40: Balance the following half-reaction occurring in
Q41: How many electrons are transferred in the
Q42: Which of the following is true
Q43: For the electrochemical cell Zn(s)| Zn<sup>2+</sup> ||
Q44: What is the value of the