Multiple Choice
What is the balanced chemical equation for the combustion of 4-methyl-1-pentene?
A) 2 C5H10(g) + 15 O2(g) 10 CO2(g) + 10 H2O( 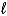 )
)
B) 2 C4H10(g) + 13 O2(g) 8 CO2(g) + 10 H2O( 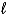 )
)
C) C4H8(g) + 6 O2(g) 4 CO2(g) + 4 H2O( 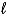 )
)
D) C6H12(g) + 9 O2(g) 6 CO2(g) + 6 H2O( 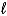 )
)
E) C5H12(g) + 13 O2(g) 5 CO2 (g) + 6 H2O( 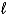 )
)
Correct Answer:

Verified
Correct Answer:
Verified
Q9: What are the two types of stereoisomers?<br>A)
Q29: In vulcanized rubber,the polymer chains of natural
Q62: Which one of the following statements concerning
Q65: What is the molecular formula of a
Q66: Formulas for derivatives of hydrocarbons may be
Q67: What class of compounds is responsible for
Q69: What is the name of the following
Q70: The carbon-carbon bonding of cyclobutane is shown
Q71: How many structural isomers possessing the alcohol
Q72: Which of the following chemical equations