Multiple Choice
Which of the following chemical equations depicts a halogenation reaction with benzene?
A) C6H6( 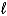 ) + 3 Br2(g) 3 C2H2Br2(g)
) + 3 Br2(g) 3 C2H2Br2(g)
B) C6H6( 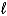 ) + Cl2(g) C6H5Cl(
) + Cl2(g) C6H5Cl( 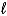 ) + HCl(g)
) + HCl(g)
C) C6H6( 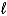 ) + CH3I(g) C6H5CH3(
) + CH3I(g) C6H5CH3( 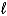 ) + HI(g)
) + HI(g)
D) C6H6( 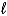 ) + Cl2(g) C6H4Cl2(
) + Cl2(g) C6H4Cl2( 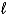 ) + H2(g)
) + H2(g)
E) C6H6( 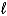 ) + 3 Br2(g) 6 C6H6Br6(g)
) + 3 Br2(g) 6 C6H6Br6(g)
Correct Answer:

Verified
Correct Answer:
Verified
Q7: Draw Lewis structures for all possible isomers
Q62: Which one of the following statements concerning
Q67: What class of compounds is responsible for
Q68: What is the balanced chemical equation
Q69: What is the name of the following
Q70: The carbon-carbon bonding of cyclobutane is shown
Q71: How many structural isomers possessing the alcohol
Q74: Four of the following compounds are
Q76: What is the name of the following
Q77: What is the major product upon addition