Multiple Choice
Which of the following is the correct bond-line structure for (CH3) 4C? 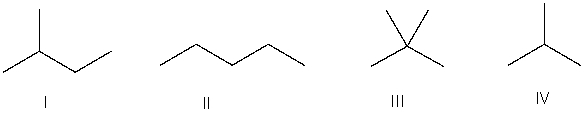
A) I
B) II
C) III
D) IV
E) None of these
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q111: Which of the following is the correct
Q112: Draw a bond-line structure for each constitutional
Q113: For the following equation, how many hydrogen
Q114: Provide a condensed structure for the following
Q115: How many lone pairs of electrons are
Q117: How many hydrogen atoms are connected to
Q118: Provide the curved arrow(s) to draw a
Q119: Draw two resonance structures for HN<sub>3</sub>.
Q120: The lone pair on oxygen in the
Q121: Draw the resonance hybrid of C<sub>6</sub>H<sub>6</sub>.