Multiple Choice
The lone pair on oxygen in the following compound is _______. 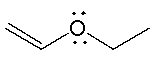
A) both localized
B) both delocalized
C) one localized and one delocalized
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q115: How many lone pairs of electrons are
Q116: Which of the following is the correct
Q117: How many hydrogen atoms are connected to
Q118: Provide the curved arrow(s) to draw a
Q119: Draw two resonance structures for HN<sub>3</sub>.
Q121: Draw the resonance hybrid of C<sub>6</sub>H<sub>6</sub>.
Q122: How many hydrogen atoms are connected to
Q123: Which of the following compounds contain an
Q124: Draw the curved arrow(s) for converting the
Q125: Draw the resonance hybrid of CH<sub>2</sub>CHCHCHCH<sub>2</sub><sup>+</sup>.