Multiple Choice
Consider the interesting structure below,called a dibromocarbene.The carbon of the dibromocarbene has one lone electron pair and two separate covalent bonds to individual bromine atoms.What is the formal charge on the carbon atom of the dibromocarbene? 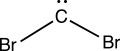
A) +2
B) +1
C) 0
D) -1
E) -2
Correct Answer:

Verified
Correct Answer:
Verified
Q2: In sum,how many total hydrogen atoms are
Q3: A carbonyl,the C <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB4360/.jpg" alt="A carbonyl,the
Q4: A C-O single bond is 143 pm
Q5: Tubulysin D is a potent cytotoxic compound
Q6: Identify which carbon atom in the molecule
Q8: To which carbon atoms in anisole would
Q9: Which would you expect to be a
Q10: The way atoms are connected to each
Q11: Which electron configuration is correct for the
Q12: Which of the following is an example