Essay
A carbonyl,the C 
O unit,is a component of many important functional groups.Consider the Lewis structures below.Convert the Lewis structures to line structures,showing all lone pairs.Indicate bond dipoles using the arrow method.Rank the structures for increasing partial positive charge.Predict which carbonyl carbon should have greatest partial positive charge,assuming that the chlorine lone pairs do not engage in resonance.Explain your answer. 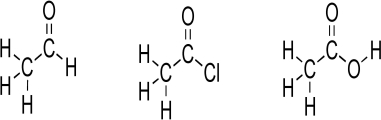
Correct Answer:

Verified
Correct Answer:
Verified
Q1: Naltrexone is an FDA-approved treatment for alcoholism
Q2: In sum,how many total hydrogen atoms are
Q4: A C-O single bond is 143 pm
Q5: Tubulysin D is a potent cytotoxic compound
Q6: Identify which carbon atom in the molecule
Q7: Consider the interesting structure below,called a dibromocarbene.The
Q8: To which carbon atoms in anisole would
Q9: Which would you expect to be a
Q10: The way atoms are connected to each
Q11: Which electron configuration is correct for the