Essay
Oxygen is an important heteroatom found in many organic molecules.Consider methanol and its protonated derivative,shown below.Indicate relevant bond dipoles using dipole arrows.How does an oxygen with a positive charge,called an oxonium species,influence the magnitude of the partial positive charge on the carbon atom? Which oxygen-carbon bond do you think is more difficult to break? Explain. 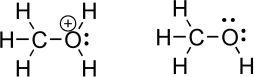
Correct Answer:

Verified
The C-O bond in the oxonium sp...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q38: (a)Sulfuric acid,H<sub>2</sub>SO<sub>4</sub>,is an important strong oxo acid
Q39: Global warming and ozone depletion both have
Q40: Which condensed formula contains a ketone?<br>A)CH<sub>3</sub>COCH<sub>3</sub><br>B)CH<sub>3</sub>CO<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub><br>C)CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>COOH<br>D)CH<sub>3</sub>CH<sub>2</sub>OH<br>E)CH<sub>3</sub>COH
Q41: Using line structures,deduce individual resonance contributors from
Q42: Draw all possible resonance forms for anisole
Q44: Using line structures,draw the individual resonance contributors
Q45: Which individual structures below could be contributing
Q46: Which carbon atom has an oxidation state
Q47: Which line structure is correct for Molecule
Q48: What is the molecular formula of this