Multiple Choice
Which individual structures below could be contributing resonance structures to the given hybrid structure? 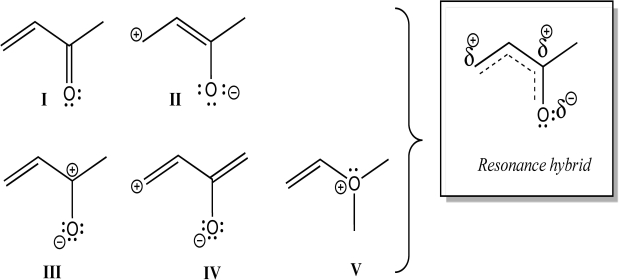
A) All are contributing structures.
B) All but V are contributing structures.
C) I, II, and III are contributing structures.
D) I, III, and IV are contributing structures.
E) Only I and III are contributing structures.
Correct Answer:

Verified
Correct Answer:
Verified
Q40: Which condensed formula contains a ketone?<br>A)CH<sub>3</sub>COCH<sub>3</sub><br>B)CH<sub>3</sub>CO<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub><br>C)CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>COOH<br>D)CH<sub>3</sub>CH<sub>2</sub>OH<br>E)CH<sub>3</sub>COH
Q41: Using line structures,deduce individual resonance contributors from
Q42: Draw all possible resonance forms for anisole
Q43: Oxygen is an important heteroatom found in
Q44: Using line structures,draw the individual resonance contributors
Q46: Which carbon atom has an oxidation state
Q47: Which line structure is correct for Molecule
Q48: What is the molecular formula of this
Q49: Which condensed formula contains a carboxylic acid?<br>A)CH<sub>3</sub>COCH<sub>3</sub><br>B)CH<sub>3</sub>CO<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub><br>C)CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>COOH<br>D)CH<sub>3</sub>CH<sub>2</sub>OH<br>E)CH<sub>3</sub>COH
Q50: Peptide bonds are the building blocks of