Short Answer
Peptide bonds are the building blocks of proteins.Consider a peptide bond formed from the amino acids alanine and serine,shown below.Draw the resonance forms and the resonance hybrid for the amide bond of the dipeptide.Use appropriate arrow notation. 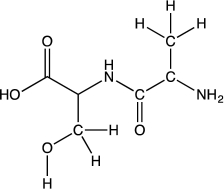
Correct Answer:

Verified
Correct Answer:
Verified
Q44: Using line structures,draw the individual resonance contributors
Q45: Which individual structures below could be contributing
Q46: Which carbon atom has an oxidation state
Q47: Which line structure is correct for Molecule
Q48: What is the molecular formula of this
Q49: Which condensed formula contains a carboxylic acid?<br>A)CH<sub>3</sub>COCH<sub>3</sub><br>B)CH<sub>3</sub>CO<sub>2</sub>CH<sub>2</sub>CH<sub>3</sub><br>C)CH<sub>3</sub>CH<sub>2</sub>CH<sub>2</sub>COOH<br>D)CH<sub>3</sub>CH<sub>2</sub>OH<br>E)CH<sub>3</sub>COH
Q51: Which of the following <font face="symbol"></font>amino acids
Q52: Which orbital does not house core electrons
Q53: Identify the molecules below as monosaccharides,disaccharides,or carbohydrates.Explain
Q54: Which electron configuration is correct for a