Multiple Choice
When applying VSEPR theory to determine the geometry about a central atom,it is important to count the number of electron groups.Separately consider the two atoms highlighted with an arrow in the molecule shown below.How many electron groups must be considered for each of these central atoms? 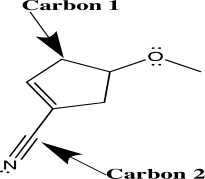
A) C1 has two groups; C2 has two groups.
B) C1 has three groups; C2 has four groups.
C) C1 has four groups; C2 has two groups.
D) C1 has four groups; C2 has three groups.
E) C1 has four groups; C2 has four groups.
Correct Answer:

Verified
Correct Answer:
Verified
Q15: Which of the following statements is true
Q16: Rank the following molecules based on increasing
Q17: A micelle is formed when soap dissolves
Q18: Which of the following functional groups contains
Q19: The carbon atoms in the molecule below
Q21: Which of the following molecules contains a
Q22: Which cycloalkane contains a C<font face="symbol"></font>C<font face="symbol"></font><font
Q23: Sodium chloride,an ionic compound,is highly water soluble
Q24: Add substituents using dash-wedge notation to achieve
Q25: Which of the following benzene derivatives would