Essay
Which of the following benzene derivatives would be most soluble in benzene? Why? 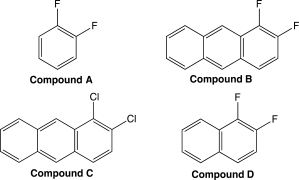
Correct Answer:

Verified
Adding extra benzene rings to the molecu...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q20: When applying VSEPR theory to determine the
Q21: Which of the following molecules contains a
Q22: Which cycloalkane contains a C<font face="symbol"></font>C<font face="symbol"></font><font
Q23: Sodium chloride,an ionic compound,is highly water soluble
Q24: Add substituents using dash-wedge notation to achieve
Q26: What is the strongest intermolecular attractive force
Q27: Rank N-N-dimethylaniline,phenethylamine,and phenethylamine hydrochloride in order of
Q28: Which cycloalkane contains a C<font face="symbol"></font>C<font face="symbol"></font><font
Q29: The solvent tert-butyl methyl ether (MTBE)is used
Q30: What is the VSEPR geometry for any