Multiple Choice
Which of the following choices correctly describes the structure of the ball-and-stick representation with the formula H3C+? 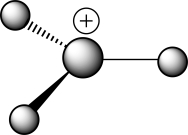
A) A carbocation with a tetrahedral carbon
B) A carbocation with trigonal planar geometry
C) A carbocation with unknown geometry
D) A carbanion with a tetrahedral carbon
E) A carbanion with trigonal planar geometry
Correct Answer:

Verified
Correct Answer:
Verified
Q29: The solvent tert-butyl methyl ether (MTBE)is used
Q30: What is the VSEPR geometry for any
Q31: Which of the following intermolecular forces is
Q32: Propanol can be dissolved in diethyl ether
Q33: Tertiary amides are typically insoluble in water.The
Q35: Which of the following molecules contains a
Q36: Your lab partner disobeyed lab rules and
Q37: Identify the weakest intermolecular force.<br>A)Hydrogen bond<br>B)Ion-dipole<br>C)Ion-ion<br>D)Dipole-induced dipole<br>E)Induced
Q38: When applying VSEPR theory to determine the
Q39: Identify the strongest intermolecular force.<br>A)Hydrogen bond<br>B)Ion-dipole<br>C)Ion-ion<br>D)Dipole-induced dipole<br>E)Induced