Multiple Choice
When applying VSEPR theory to determine the geometry about a central atom,it is important to count the total number of bonded and nonbonded electron groups.Separately consider the two atoms highlighted with an arrow in the molecule shown below.How many bonded electron groups must be considered for each of these central atoms? 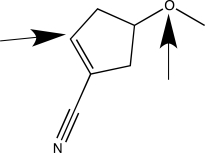
A) C has two groups; O has two groups.
B) C has three groups; O has four groups.
C) C has three groups; O has two groups.
D) C has three groups; O has three groups.
E) C has four groups; O has four groups.
Correct Answer:

Verified
Correct Answer:
Verified
Q33: Tertiary amides are typically insoluble in water.The
Q34: Which of the following choices correctly describes
Q35: Which of the following molecules contains a
Q36: Your lab partner disobeyed lab rules and
Q37: Identify the weakest intermolecular force.<br>A)Hydrogen bond<br>B)Ion-dipole<br>C)Ion-ion<br>D)Dipole-induced dipole<br>E)Induced
Q39: Identify the strongest intermolecular force.<br>A)Hydrogen bond<br>B)Ion-dipole<br>C)Ion-ion<br>D)Dipole-induced dipole<br>E)Induced
Q40: Turn the original molecule shown below 90°
Q41: Which cycloalkane has the greatest ring strain
Q42: Dimethyl sulfoxide (DMSO)is a polar aprotic solvent
Q43: What is the VSEPR geometry for the