Multiple Choice
Turn the original molecule shown below 90° in a clockwise direction on the plane of this paper.Which choice represents the product of this manipulation? 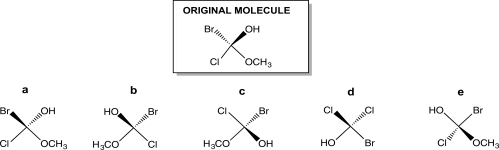
A) Structure a
B) Structure b
C) Structure c
D) Structure d
E) Structure e
Correct Answer:

Verified
Correct Answer:
Verified
Q35: Which of the following molecules contains a
Q36: Your lab partner disobeyed lab rules and
Q37: Identify the weakest intermolecular force.<br>A)Hydrogen bond<br>B)Ion-dipole<br>C)Ion-ion<br>D)Dipole-induced dipole<br>E)Induced
Q38: When applying VSEPR theory to determine the
Q39: Identify the strongest intermolecular force.<br>A)Hydrogen bond<br>B)Ion-dipole<br>C)Ion-ion<br>D)Dipole-induced dipole<br>E)Induced
Q41: Which cycloalkane has the greatest ring strain
Q42: Dimethyl sulfoxide (DMSO)is a polar aprotic solvent
Q43: What is the VSEPR geometry for the
Q44: Rank 1,4-dimethylbenzene,phenol,and N,N-dimethylaniline in order of decreasing
Q45: Which functional group will engage in dipole-dipole