Multiple Choice
How does the presence of the lone pair affect the geometry of the central atom in the following molecule? 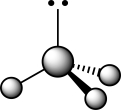
I The lone pair is attracted to the nuclei of the three substituents,creating larger bond angles.
II The lone pair repels the three sets of covalently bonded electrons.
III The lone pair has no bearing whatsoever on the VSEPR geometry at the central atom.
IVThe bond angles are smaller than a traditional tetrahedral bond angle due to lone pair repulsion.
A) I
B) II
C) III
D) IV
E) II and IV
Correct Answer:

Verified
Correct Answer:
Verified
Q43: What is the VSEPR geometry for the
Q44: Rank 1,4-dimethylbenzene,phenol,and N,N-dimethylaniline in order of decreasing
Q45: Which functional group will engage in dipole-dipole
Q46: Are any of the four 1,2-difluorocyclopropane isomers
Q47: Consider the structure of sodium benzoate,NaOC(O)Ph,the sodium
Q48: When mixed,which of the following pairs of
Q50: Which of the following molecules contains a
Q51: Which of the following molecules possesses at
Q52: How many hydrogen-bond donors and acceptors are
Q53: What is the strongest intermolecular attractive force