Multiple Choice
When mixed,which of the following pairs of compounds will exhibit both ion-dipole and ion-ion intermolecular attractive forces? 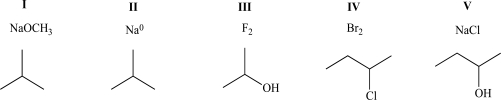
A) I
B) II
C) III
D) IV
E) V
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q43: What is the VSEPR geometry for the
Q44: Rank 1,4-dimethylbenzene,phenol,and N,N-dimethylaniline in order of decreasing
Q45: Which functional group will engage in dipole-dipole
Q46: Are any of the four 1,2-difluorocyclopropane isomers
Q47: Consider the structure of sodium benzoate,NaOC(O)Ph,the sodium
Q49: How does the presence of the lone
Q50: Which of the following molecules contains a
Q51: Which of the following molecules possesses at
Q52: How many hydrogen-bond donors and acceptors are
Q53: What is the strongest intermolecular attractive force