Multiple Choice
How many electrons are in the following molecule's largest conjugated π system? 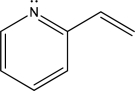
A) Two
B) Four
C) Six
D) Eight
E) Ten
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q38: How many nodes does the following atomic
Q39: Identify the areas of constructive interference for
Q40: Which molecular orbital has the lowest energy?<br>A)The
Q41: Which of the following reactions releases the
Q42: How many electrons are in the given
Q44: Use the Frost method to explain whether
Q45: How many different π systems does the
Q46: Which is more acidic,H<sub>A</sub> or H<sub>B</sub>? Explain.
Q47: Which one of the following compounds is
Q48: Which hydrogen has the lowest Pk<sub>a</sub>? <img