Essay
Use the Frost method to explain whether furan,diagramed below is aromatic,antiaromatic,or nonaromatic.In your Frost diagram,fill in all π electrons,and label the HOMO and the LUMO. 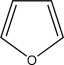
Correct Answer:

Verified
Furan is aromatic,be...View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Correct Answer:
Verified
View Answer
Unlock this answer now
Get Access to more Verified Answers free of charge
Q39: Identify the areas of constructive interference for
Q40: Which molecular orbital has the lowest energy?<br>A)The
Q41: Which of the following reactions releases the
Q42: How many electrons are in the given
Q43: How many electrons are in the following
Q45: How many different π systems does the
Q46: Which is more acidic,H<sub>A</sub> or H<sub>B</sub>? Explain.
Q47: Which one of the following compounds is
Q48: Which hydrogen has the lowest Pk<sub>a</sub>? <img
Q49: Which term can be used to define