The Compound RX3 Decomposes According to the Equation
3RX3 \(\To\0 R + R2X3 + 3X2
In an Experiment
Multiple Choice
The compound RX3 decomposes according to the equation
3RX3 \(\to\0 R + R2X3 + 3X2
In an experiment the following data were collected for the decomposition at 100°C.What is the average rate of reaction over the entire experiment?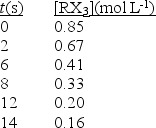
A) 0.011 mol L-1s-1
B) 0.019 mol L-1s-1
C) 0.044 mol L-1s-1
D) 0.049 mol L-1s-1
E) 0.069 mol L-1s-1
Correct Answer:

Verified
Correct Answer:
Verified
Q9: The units of the rate of reaction
Q18: Is a bimolecular reaction necessarily second-order? Is
Q21: Consider the following mechanism for the
Q25: The rate law for the rearrangement
Q27: Consider the following mechanism for the
Q31: Which one of the following sets of
Q31: The reaction A <span class="ql-formula"
Q60: When the reaction A <font face="symbol"></font> B
Q60: The decomposition of dinitrogen pentaoxide to nitrogen
Q88: The rate constant for the reaction