Multiple Choice
Write the mass-action expression,Qc,for the following chemical reaction equation.
2C6H6(g) + 15O2(g) 
12CO2(g) + 6H2O(g)
A) 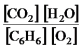
B) 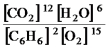
C) 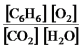
D) 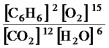
E) 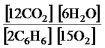
Correct Answer:

Verified
Correct Answer:
Verified
Q14: The two equilibrium constants for the same
Q69: The reaction of nitrogen with oxygen
Q70: What is the mass-action expression,Q<sub>p</sub>,for the following
Q71: What is the mass-action expression,Q<sub>c</sub>,for the following
Q72: At 500°C the equilibrium constant,K<sub>p</sub>,is 4.00
Q73: Methanol can be synthesized by combining carbon
Q75: The following reaction is at equilibrium at
Q76: Consider the equilibrium reaction: N<sub>2</sub>O<sub>4</sub>(g) <img
Q77: Ethane can be formed by reacting acetylene
Q79: The equilibrium constant K<sub>c</sub> for the reaction<br>PCl<sub>3</sub>(g)+