Multiple Choice
What is the mass-action expression,Qc,for the following chemical reaction?
4H3O+(aq) + 2Cl-(aq) + MnO2(s) 
Mn2+(aq) + 6H2O(l) + Cl2(g) -
A) 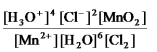
B) 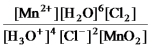
C) 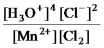
D) 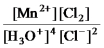
E) None of these expressions is correct.
Correct Answer:

Verified
Correct Answer:
Verified
Q66: The reaction system<br>CS<sub>2</sub>(g)+ 4H<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5832/.jpg" alt="The
Q67: The equilibrium constant K<sub>c</sub> for the reaction<br>A(g)+
Q68: Nitric oxide and bromine were allowed
Q69: The reaction of nitrogen with oxygen
Q70: What is the mass-action expression,Q<sub>p</sub>,for the following
Q72: At 500°C the equilibrium constant,K<sub>p</sub>,is 4.00
Q73: Methanol can be synthesized by combining carbon
Q74: Write the mass-action expression,Q<sub>c</sub>,for the following chemical
Q75: The following reaction is at equilibrium at
Q76: Consider the equilibrium reaction: N<sub>2</sub>O<sub>4</sub>(g) <img