Multiple Choice
Write the mass-action expression,Qc,for the following chemical reaction.
Zn(s) + 2Ag+(aq) 
Zn2+(aq) + 2Ag(s)
A) 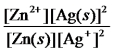
B) 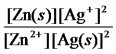
C) 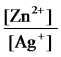
D) 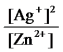
E) 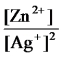
Correct Answer:

Verified
Correct Answer:
Verified
Q32: Changing the amount of reactant or product
Q41: Write the mass-action expression,Q<sub>c</sub>,for the following chemical
Q42: The reaction of nitric oxide to form
Q43: Write the mass-action expression,Q<sub>c</sub>,for the following chemical
Q44: Consider the following two equilibria and their
Q45: The following reaction is at equilibrium in
Q47: Consider the equilibrium<br>H<sub>2</sub>(g)+ Br<sub>2</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5832/.jpg" alt="Consider
Q48: A container was charged with hydrogen,nitrogen,and ammonia
Q49: Ammonium iodide dissociates reversibly to ammonia and
Q50: The reaction system<br>POCl<sub>3</sub>(g) <img src="https://d2lvgg3v3hfg70.cloudfront.net/TB5832/.jpg" alt="The reaction