Multiple Choice
Write the mass-action expression,Qc,for the following chemical reaction.
Fe3+(aq) + 3OH-(aq) 
Fe(OH) 3(s)
A) 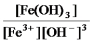
B) 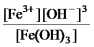
C) 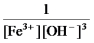
D) 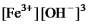
E) 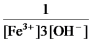
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q36: Which of the following has an effect
Q37: The equilibrium constant,K<sub>p</sub>,has a value of
Q39: Methanol can be synthesized by combining carbon
Q40: Nitric oxide is formed in automobile exhaust
Q42: The reaction of nitric oxide to form
Q43: Write the mass-action expression,Q<sub>c</sub>,for the following chemical
Q44: Consider the following two equilibria and their
Q45: The following reaction is at equilibrium in
Q46: Write the mass-action expression,Q<sub>c</sub>,for the following chemical
Q74: Although a system may be at equilibrium,