Multiple Choice
Consider the reactions of cadmium with the thiosulfate anion.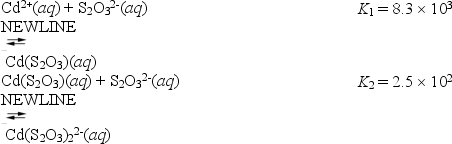
What is the value for the equilibrium constant for the following reaction?
Cd2+(aq) + 2S2O32-(aq) 
Cd(S2O3) 22-(aq)
A) 0.030
B) 33
C) 8.1 103
D) 8.6 103
E) 2.1 106
Correct Answer:

Verified
Correct Answer:
Verified
Q26: When 0.152 mol of solid PH<sub>3</sub>BCl<sub>3</sub>
Q27: Compounds A,B,and C react according to the
Q28: About half of the sodium carbonate
Q29: Sodium hydrogen carbonate decomposes above 110°C to
Q30: At 850°C,the equilibrium constant K<sub>p</sub> for the
Q32: Hydrogen bromide will dissociate into hydrogen and
Q33: Write the expression for K<sub>c</sub> and K<sub>p</sub>
Q34: Nitrogen dioxide decomposes according to the
Q35: Ammonia is synthesized in the Haber
Q36: Which of the following has an effect