Multiple Choice
About half of the sodium carbonate produced is used in making glass products because it lowers the melting point of sand,the major component of glass.When sodium carbonate is added to water it hydrolyses according to the following reactions.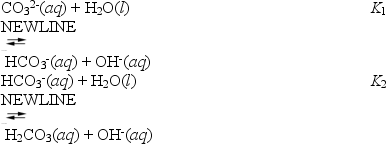
These can be combined to yield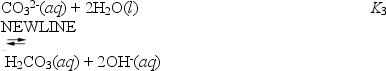
What is the value of K3?
A) K1 K2
B) K1 K2.
C) K1 + K2
D) K1 - K2
E) (K1K2) 2
Correct Answer:

Verified
Correct Answer:
Verified
Q23: a.State Le Chatelier's principle<br>b.The following reaction is
Q24: The following reaction is at equilibrium at
Q25: At 25°C,the equilibrium constant K<sub>c</sub> for
Q26: When 0.152 mol of solid PH<sub>3</sub>BCl<sub>3</sub>
Q27: Compounds A,B,and C react according to the
Q29: Sodium hydrogen carbonate decomposes above 110°C to
Q30: At 850°C,the equilibrium constant K<sub>p</sub> for the
Q31: Consider the reactions of cadmium with
Q32: Hydrogen bromide will dissociate into hydrogen and
Q33: Write the expression for K<sub>c</sub> and K<sub>p</sub>