Multiple Choice
Nitric oxide reacts with chlorine to form NOCl.The data refer to 298 K.
2NO(g) + Cl2(g) 2NOCl(g) 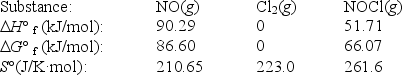
What is the value of G° for this reaction at 550 K?
A) -143.76 kJ
B) -78.78 kJ
C) -22.24 kJ
D) -10.56 kJ
E) 66,600 kJ
Correct Answer:

Verified
Correct Answer:
Verified
Related Questions
Q8: For a given reaction, a change in
Q43: Which of the following is true for
Q49: For what signs of <font face="symbol"></font>H and
Q58: In some spontaneous processes, the entropy of
Q63: Which of the following results in a
Q69: Consider the figure below which shows
Q70: The temperature at which the following process
Q71: Use the given data at 298
Q75: Hydrogen sulfide decomposes according to the
Q102: When a sky diver free-falls through the